Water shortages and inhibited access to fresh water all over the world have inspired a surge in wastewater recycling, but there are many other benefits to recycling waste water. Untreated waste water is dumped into the Earth’s oceans at alarming rates. This pollution causes oxygen depletion and disease that kill marine wildlife. By recycling waste water, we can reduce the amount of water dumped into oceans and improve the quality of the water that is dumped.
Industrial waste water can have a significant affect on operational costs, including transportation expenses for hauling waste water out and fresh water in and the cost of the fresh water itself. On-site waste water recycling eliminates waste water transportation costs and drastically reduces the amount of fresh water needed.
A complex process is necessary in order to recycle domestic and industrial sewage. The water first has to go through a multi-step waste water treatment system and then on to a drinking water treatment system, if it’s going to be reused before being reintroduced into the environment. Part of the process requires the use of mineral filtration media like anthracite and manganese.
The Urban Water Cycle
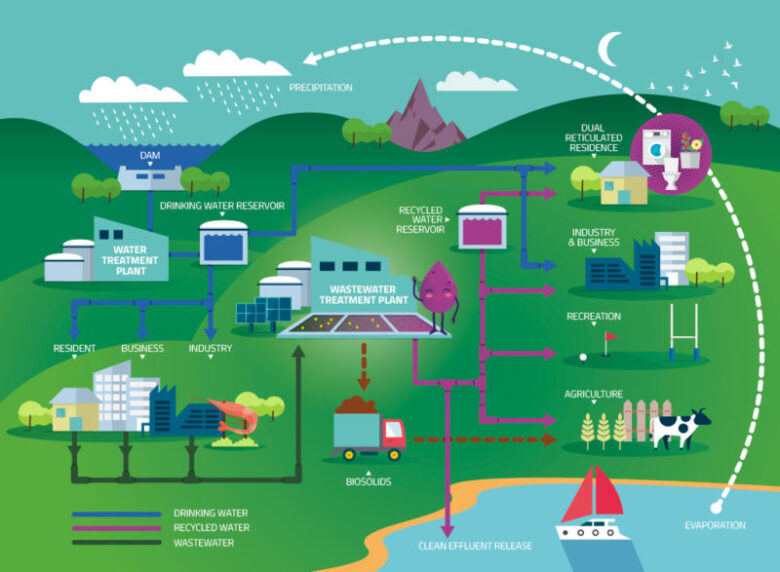
Waste water, also called sewage or effluent, first enters a waste water treatment facility. Foreign objects and debris are removed from the water in the first stage of treatment. Everything from toilet tissue to articles of clothing and children’s toys are extracted during debris removal.
The waste water is then sent to a tank where sediment is allowed to collect at the bottom and fats and oils rise to the top. A skimmer removes the sediment and oils, which are sent off for further treatment.
In the next tank, microorganisms are introduced to the waste water and begin to break down organics, solids, soaps, solids, sugars, and proteins. Once the resulting sludge is removed, the water is carried on to another tank and the microorganisms are separated and returned to their original tank. A disinfectant is added to the effluent and it’s sent on for further treatment.

Before treated waste water can be put back into the municipal supply or environmental source, it has to go through a second facility specifically for drinking water treatment. It is generally mixed with non-waste water before entering the drinking water treatment facility.
Sulphuric acid and ferric chloride are added to the water to lower pH and begin the coagulation of particles. The water is stirred to encourage flocculation, and the flocs are removed by a skimmer. Ozone, created by electrifying liquid oxygen, is added to the water for initial disinfection. Sodium bisulfite removes the ozone.
The water passes through a set of filters that remove small particles. This is where mineral filtration takes place. Generally, these filters will use anthracite, greensand, or activated carbon to achieve a thorough purification. Another round of disinfection is done with chlorine and the water is returned to an adequate pH by the addition of sodium hydroxide.
A final round of disinfection is performed using ultraviolet lights. Fluoride is added to help prevent tooth decay and orthophosphate may be added to reduce corrosion that causes lead contamination from old pipes.
Mineral Filtration
Mineral filtration is typically done using anthracite, activated carbon or manganese greensand. Manganese greensand and anthracite can be used together in a dual-media filter while activated carbon or biologically activated carbon would be used alone. Click here to read more.
Anthracite

Anthracite is the coal of high hardness that is used in single or dual-media filtration. Its irregularly-shaped particles don’t pack down like standard filter sand, making it more efficient at filtering large particles. This arrangement allows for longer filter runs and less frequent backwashing because all the trapped particles are not stuck in the upper portion of the filter. Anthracite can be used as an upper bed over manganese greensand.
Manganese Greensand

Manganese greensand is created through a controlled process that attaches a manganese oxide coating to grains of glauconitic greensand. This compound is used to remove iron, manganese, and arsenic from the water before it re-enters the municipal supply or returns to the environmental source. Manganese greensand oxidizes manganese and reduces potassium permanganate. The grains are the perfect size and shape to trap precipitates of iron and manganese passing through an upper bed of anthracite.
Activated Carbon
Activated carbon is a highly porous material that adsorbs organic compounds, taste and odour compounds, and chemicals. It offers a large surface area for adsorption. Activated carbon is great for removing chlorine, sediment, and volatile organic compounds.


