Batteries have become a very important part of the human world. We need the use of batteries in our everyday lives. We need them for our phones, our remote controllers, fossil fuel cars and now even bigger and better batteries are needed for the up-and-coming future of electric cars.
Lithium-ion batteries first came as a concept in the 1970s and then were developed by John Goodenough in the 1980s. Li-ion batteries are a type of rechargeable batteries that are important in everyday use for portable electronics and vehicles, but they are also very important for airplanes and even military applications.
Construction
The three main components of a Li-Ion battery are the negative and positive electrolyte and electrodes. Usually, the negative is made from carbon while the positive electrolyte is a lithium salt in some organic solvent and the electrode is a metal oxide. The roles of these electrodes reverse between cathode and anode, depending on the direction of the current flow.
Depending on the choice of materials, energy density, voltage, safety and life of a Li-Ion battery can change dramatically. Recently, nanotechnology has shown to be of great use to improve a battery’s performance.
Shapes
Lithium-ion batteries are available in a variety of shapes which are usually divided into 4 groups
• Small and cylindrical
• Large and cylindrical
• Flat (like those which are usually used in cell phones or laptops)
• Large threaded terminals in a rigid plastic case
Cells with the cylindrical shape are usually made with the “jelly roll” design in mind. This design is in the form of a long “sandwich” of a negative electrode, two separators and a negative electrode all rolled into one cylindrical casing. However, the disadvantage of this method is that the cells will have a high series of inductance.
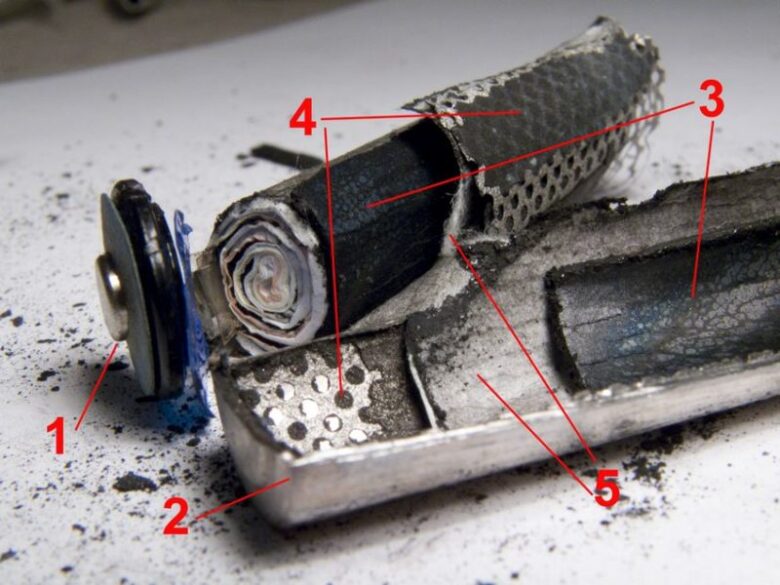
This is why flat or pouch cells (those used in phones) have the highest gravimetric energy density. Although, they still need some kind of containment to prevent the dangers of expansions because their SOC or state-of-charge is very high and for general structural stability. Both pouch-style (flat) and rigid plastic battery cells are sometimes referred to as prismatic cells because of their rectangular shapes.
If you are looking to test the safety of a lithium-ion battery whether it is from a smartphone, laptop, or any kind of portable device we recommend you check out ugtx.com.
Testing and safety

Li-Ion batteries can be dangerous and a safety hazard because they contain a flammable electrolyte and could pressure themselves when they are damaged. A pressurized battery should be immediately discarded since it is now a fire hazard that could even explode. A battery cell could also cause a fire or even an explosion if it is charged too quickly.
This is why testing standards have become much higher for Li-Ion batteries. To satisfy these new standards, you need to follow a range of rules for the testing conditions and battery-specific tests. Lithium-ion must also be properly regulated when being transported since any kind of overheating or crushing may cause a cell rupture which could result in a fire.
To reduce any kind of risks of fire, leakage or explosions, many Li-Ion batteries contain a fail-safe “switch” that disconnects the battery when the voltage goes out of the 3.1-4.3 V safe zone.


